You Read the Buret Level With Your Eye Well Below the Meniscus. The Resulting Buret Reading Will Be
Volumetric Glassware
In quantitative chemistry, it is often necessary to make volume measurements with an mistake on the club of 0.one%, i function per thou. This involves using glassware that can incorporate or deliver a volume known to a few hundredths of a milliliter, or about 0.01 mL. I tin then report quantities greater than 10 mL to iv significant figures. Glassware designed for this level of accuracy and precision is expensive, and requires some intendance and skill to requite best results. 4 main types of volumetric glassware are common: the graduated cylinder, the volumetric flask, the buret and the pipet. These have specific uses and volition exist discussed individually. At that place are some points that are common to all types, however. These involve cleanliness and how to read volumes accurately. Cleanliness is essential to adept results. Chemically make clean glass supports a uniform film of h2o, with no hanging droplets visible. Rinse your glassware thoroughly with deionized water when you are finished with it. If you are suspicious at all, launder information technology earlier yous use it equally well. With some types of glassware, ane "conditions" the apparatus past rinsing with a few small-scale portions of the solution one volition be measuring prior to conducting the bodily work. This prevents h2o droplets from diluting one'due south solution, and changing the concentration. More than detail on how to practise this volition be given in the word of the individual pieces of glassware. All volumetric glassware is calibrated with markings used to determine a specific volume of liquid to varying degrees of accuracy. To read this volume exactly, the bottom of the curved surface of the liquid, the meniscus, should be located at the scribed line for the desired volume. It is often easier to see the meniscus if y'all put a white paper or card behind the appliance. If your centre is above or below the level of the meniscus, your readings will be inaccurate due to the miracle of parallax. View the meniscus at a level perpendicular to your center to avoid this as a source of error.
TC Versus TD
Some volumetric glassware bears the label " TC 20°C" which stands for " to contain at 20°C." This means that at twenty°C, that flask will have precisely the volume listed inside it. If you were to pour out the liquid, y'all would need to get every drop out of it to have that volume. Alternatively, some volumetric glassware bears the characterization " TD xx°C" which stands for " to deliver at twenty°C." This means that at 20°C, precisely the volume listed will go out it when the contents are allowed to bleed out of the vessel. It is non necessary to get every last drop and, in fact, it is inaccurate to blow the last bit out of a volumetric pipet.
Graduated Cylinders
Most students are familiar with graduated cylinders, which are used to measure and dispense known volumes of liquids. They are manufactured to contain the measured volume with an error of 0.v to one%. For a 100 mL graduated cylinder, this would be an error of 0.five to i.0 mL. Measurements fabricated with a graduated cylinder can be reported to 3 meaning figures.
Figure i
Volumetric Flasks
Watch the movie on using a volumetric flask. The volumetric flask, bachelor in sizes ranging from 1 mL to 2 L, is designed to contain a specific book of liquid, usually to a tolerance of a few hundredths of a milliliter, virtually 0.i% of the flask's capacity. The flask has a calibration line engraved on the narrow part of its cervix. It is filled with liquid and then the bottom of the meniscus is on this engraved line. The calibration line is specific to a given flask; a set of flasks congenital to contain the same volume will have lines at different positions.
Figure 2
Volumetric flasks are used to make solutions with very accurately known concentrations. There are two ways to do this. One tin kickoff with a solid solute or with a concentrated stock solution. When working with a solid solute, one weighs the material to the desired accurateness and transfers it carefully and completely to the volumetric flask. If solute is lost in transfer, the actual concentration of the resulting solution will be lower than the calculated value. Therefore, one weighs the solid in a beaker or other glassware that can be rinsed with the solvent, typically h2o, and transfers it into the flask. Additional solvent is added, but not enough to fill the wide part of the flask. The solute is dissolved past swirling the flask, or past stoppering it and inverting it repeatedly. In one case the solute is dissolved, more than solvent is added to bring the volume to the marking on the flask. The last portion should be added very carefully, dropwise, then the lesser of the meniscus is at the marker. The flask is then stoppered and inverted a few times to completely mix the solution. When diluting a stock solution, the desired book of solution is transferred into the flask via a pipet. The solvent is then added as described above. Plainly, the concentration of the stock solution must be accurately known to as many significant figures as ane desires for the dilute solution. Also, the volume transferred must exist known to the desired number of significant figures. Never make full a volumetric flask with solvent and then add together solute. This results in overfilling the flask, and the volume will not be known accurately. It is sometimes useful to have some solvent in the flask before adding the solute. This is a good practise when dealing with volatile solutes. Volumetric flasks are not used for storage of solutions. Once the solution is prepared, it is transferred to a make clean, labeled bottle or beaker. The flask is then done and rinsed well. The last few rinsings should exist with deionized h2o
Burets
A buret is a long, narrow tube with a stopcock at its base of operations. It is used for accurately dispensing variable volumes of liquids or solutions. It is graduated in 0.1 mL increments, with the 0.00 mL mark at the top and the 50.00 mL mark almost the bottom. Notice that the marks do not go all the way to the stopcock. Therefore the buret actually will hold more than 50.00 mL of solution. Burets with liquid capacities of 25.00 mL and 10.00 mL are besides available.

Figure 3
Watch the picture on cleaning and conditioning a buret. For optimal accuracy and to prevent contamination, a buret must be clean. To exam a buret for cleanliness, close its stopcock and cascade a small volume (5-10 mL) of deionized water into it. Hold the buret at a slant, near parallel to the desk surface. Slowly rotate the buret and allow the liquid to glaze its within surface. Then hold it upright; the liquid should settle to the bottom of the buret in sheets, leaving no droplets on the interior walls. If droplets course on the walls, wash the inside with a soap solution, and rinse with distilled or deionized h2o. Echo the cleanliness test. Merely before use, a buret should be "conditioned" to ensure that any water adhering to the inside walls is removed. Add ~v mL of the liquid that is to be used into the buret. Rinse the walls of the buret, and then bleed the liquid through the stopcock. Echo with a second volume of liquid. The buret can now be filled with solution. Practice this carefully and avoid trapping air bubbling in the tube. Yous may need a small funnel. The liquid level can be in a higher place the 0.00-mL mark. Clamp the filled buret in place if this was not done prior to filling; it is sometimes easier to hold the buret while filling it. Open the stopcock and drain plenty liquid to fill the buret'southward tip. Have a chalice for waste product solution handy for this and like operations. There should be no bubbles in the tube or tip of the buret. These will lead to volume errors. If there are bubbling in the tube, carefully tap the buret to gratuitous them. Utilize the stopcock to strength bubbles out of the tip. Information technology may exist necessary to empty and refill the buret. Watch the movie on titration. When the buret is clean and bubble-complimentary, bleed the liquid until the meniscus (the bottom of the curved surface of the liquid) is at or slightly below the 0.00-mL mark. Information technology is not necessary to align the meniscus exactly at the 0.00-mL mark since the departure between the initial and final volumes is the desired measurement. If in that location is a drop of liquid clinging to the buret tip, remove information technology by gently touching the tip to a drinking glass surface, such as the edge of the waste beaker or wiping with a Kimwipe. The volume of a drop is about 0.i mL, the same volume as the buret's graduations. Observe the bottom of the meniscus, and read the liquid level in the buret to the nearest 0.01 mL at that point. This volition take a little exercise. Remember, yous are reading from the top downwards. Record this value as the initial book. Although information technology is tricky to "read between the lines," remember that the concluding digit of a measurement is expected to take some uncertainty! One-5th (one/5) of a division (0.02 mL) tin be reproducibly estimated if the meniscus is between calibration marks, later a little practice. Now dispense the liquid you need. If y'all are using the buret to measure a set amount of liquid, determine what the final reading should be to obtain that corporeality. Manipulate the liquid slowly into the receiving vessel. Remember, in a make clean buret, water will glaze the interior walls and drain slowly. Afterward closing the stopcock catch whatever hanging droplet in the receiving vessel. Information technology is function of the measurement at this point, then do not catch it in the waste matter container. Expect a few seconds for the meniscus to stabilize, then read and record the final book to the nearest 0.01 mL. The departure between the initial and final readings is the volume you lot dispensed. When using a buret, information technology is easier to piece of work with the exact volume dispensed than to try to manipulate an exact volume. Plan your piece of work with this in mind. Although burets are sometimes used as dispensers, they are far more often used in procedures called titrations. In a titration, one attempts to decide an equivalence point as exactly every bit possible. This usually involves the offset persistent color alter of an indicator. With a little practice, one can dispense fractions of drops (less than 0.1 mL) into the titration vessel, and reproduce results within 0.10 mL or less. Watch the movie on cleaning a buret. When finished using a buret, drain the remaining liquid and make clean information technology advisedly. Stop with several rinses of deionized h2o including the stopcock and tip. If solute dries in the buret, it can be very difficult to remove. Clamp the buret in the buret clamp upside down with the stopcock open up and then that it will dry for the next lab session.
Pipets
Spotter the movie on pipeting techniques. Pipets are designed to evangelize a known volume of a liquid. Their volumes range from less than 1 mL to near 100 mL. There are several types, which vary in accuracy and in the type of task for which they are optimum.
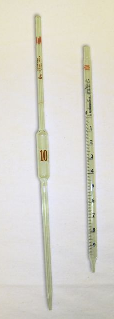
Figure 4
- Volumetric pipets are meant to hold a single, specific volume. This blazon of pipet is a narrow tube with a "bubble" in its center, a tapered tip for delivery of liquid, and a single graduation marking nearly the top (opposite the tapered cease) of the tube. Volumetric pipets, sometimes called transfer pipets, are the near accurate pipets. They generally deliver the specified volume ±0.i%, an error of a few hundredths of a milliliter.
- Most volumetric pipets are marked TD (to deliver) and are drained by gravity. If a driblet remains on the tip of the pipet, information technology is touched gently to the receiving vessel to draw off the remaining liquid or wipe with a Kimwipe. This type of pipet is not designed have residual liquid forced out by bravado.
- Mohr pipets, also called measuring pipets, are straight tubes with graduations (usually at 0.ten-mL intervals) and a tapered end. Mohr pipets are not designed to be drained completely. The operator fills them to a certain level, then dispenses the desired amount of liquid. They are much like burets and tin can be used for small book titrations. This takes a fair amount of practice, though.
- Serological pipets are a hybrid of the two previous types. Like Mohr pipets, they are straight tubes with graduations. They can be almost every bit accurate as volumetric pipets, and they are very convenient. They tin can be used to manipulate various volumes. For instance, an experiment may call for dilutions of a stock solution, requiring 2.5, 5.0, and 7.5 mL of solution. A serological pipet is an splendid tool for this sort of work. About serological pipets are calibrated TD/Accident Out. They have a shaped tip, to hold a cotton plug, and horizontal bands nigh the top of the tube. They are drained by gravity, and the last drop is gently blown out with a pipet bulb into the receiving vessel.
Earlier apply, a pipet should be rinsed a few times with deionized water. If h2o droplets remain on the inside, try cleaning the pipet with warm soap solution followed by several rinses of deionized water. A pipet should be "conditioned" after cleaning. Showtime, obtain a small volume of the solution to exist dispensed in a beaker or flask. Never pipet straight from the stock solution canteen! Since you may contaminate this solution, plan on discarding it after conditioning is complete. Describe a small book of the solution to be dispensed into the pipet, then turn the pipet sideways (parallel to the demote height) and slowly rotate it to coat the within surface. Then let the solution to completely drain. The pipet is at present ready for transfers of the desired liquid. Filling a pipet takes a little practice; y'all may want to endeavour it a few times with deionized water after cleaning it. Use a pipet seedling—never your rima oris!—for this purpose. The bulb has a tapered rubber seal. It should never be fitted tightly onto the top of the pipet. Concord the bulb against the elevation of the tube, just tightly enough to get a seal. Clasp and hold the seedling in the compressed form, lower the tip of the pipet into the solution of interest, and slowly release the pressure on the bulb. When the liquid has risen slightly to a higher place the calibration mark on the neck, quickly remove the seedling and place a finger (typically a thumb or an alphabetize finger) firmly on the top of the pipet. A gentle rocking or twisting motion of your finger should allow the solution to bleed until the lesser of the meniscus rests at the calibration marking. Remove any droplet hanging on the tip by gently touching the tip to a glass surface, such as a chalice for waste material solution. The contents of the pipet can now be tuckered into the desired container. Motion the tip of the pipet into the container, remove your finger, and allow the liquid to flow out of the pipet. A volumetric pipet volition take one remaining drop that should be "touched off" by gently touching the tip of the pipet to an within edge of the container. A small volume of liquid will remain in the pipet and should be left in that location. Serological pipets should have all liquid in the pipet expelled—typically with a slight pressure from the rubber bulb. Graduated pipets (serological or Mohr) are a little trickier to utilise than volumetric pipets, because at that place are more than options in filling and reading them. Examine such a pipet before you use information technology and retrieve through what you will do with it. Many graduated pipets take two scales. 1 calibration has the highest values toward the dispensing tip, and is read similar a buret. The other has the lowest values virtually the dispensing tip. This is easier to read when cartoon liquid into the pipet for transfer to some other vessel. After using a pipet, rinse it several times with deionized h2o. Draw up its full volume and allow it to bleed. If you apply the pipet repeatedly for several aliquots (samples) of the same solution, do not rinse the pipet between uses. You will just have to condition it each fourth dimension. Clean it when you are finished, or before yous start working with a unlike solution.
Meaning Figures and Volumetric Glassware
Every bit the preceding give-and-take points out, most volumetric glassware is accurate to a few hundredths of a milliliter and is designed and then a careful operator can reproduce measurements to this degree of precision. Therefore, measurements made with volumetric glassware are reported to 0.01 mL. Depending on the volumes used, iii or 4 significant figures can be shown in data tables and carried in calculations.
You Read the Buret Level With Your Eye Well Below the Meniscus. The Resulting Buret Reading Will Be
Source: https://www.webassign.net/question_assets/tccgenchem2l1/glassware/manual.html